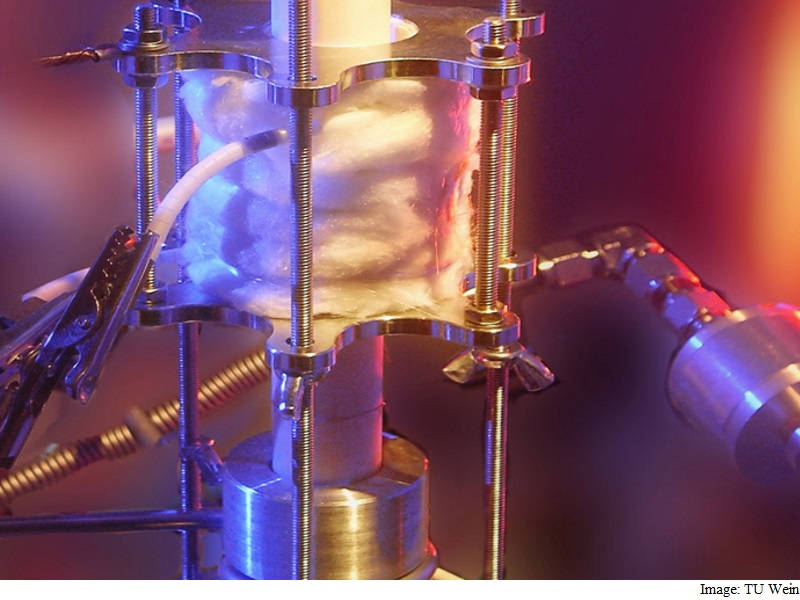
A team of scientists has developed a way to chemically store solar energy. The team designed a photo-electrochemical cell that does this amazing feat even at high temperatures.
There are numerous ways of storing energy from the sun. Plants naturally can absorb sunlight and store its energy chemically.
However, imitating this on a large industrial scale is difficult. Photovoltaics on the other hand can convert the energy from sunlight to electricity, but the efficiency of the solar cells at high temperatures decreases.
The researchers from the Vienna University of Technology (TU Wien) were able to solve this problem by combining high temperature photovoltaics with an electrochemical cell. This allows ultraviolet light to be directly used to pump oxygen ions through a solid oxide electrolyte and the energy of the UV light is stored chemically.
“This would allow us to concentrate sunlight with mirrors and build large-scale plants with a high rate of efficiency”, said Georg Brunauer a member of the team.
The team managed to assemble a cell by using special metal oxides – so-called perovskites – instead of the ordinary silicon based photovoltaics.
“Our cell consists of two different parts – a photoelectric part on top and an electrochemical part below. In the upper layer, ultraviolet light creates free charge carriers, just like in a standard solar cell,” he added.
The electrons in this layer are immediately removed and travel to the bottom layer of the electrochemical cell.
Once there, these electrons are used to ionize oxygen to negative oxygen ions, which can then travel through a membrane in the electrochemical part of the cell.
“This is the crucial photoelectrochemical step, which we hope will lead to the possibility of splitting water and producing hydrogen,” Brunauer noted.
In its first evolution step, the photoelectrochemical cell works as a UV-light driven oxygen pump. It yields an open-current voltage of up to 920 millivolts at a temperature of 400 degrees Celsius.
The cell was recently presented in the Advanced Functional Materials journal. It looks promising and will solve a lot of energy problems if perfected.